キーワード:医工連携、生体材料、レギュラトリーサイエンス
 私たちの病気は、大きく分けて、投薬による内科的治療と手術による外科的治療の2つで治療されます。多くの画期的な新薬や治療器具が米国を中心とした大学の自由な風土を土壌に誕生していることを追い風に、日本でも大学の研究成果に礎を置く創薬系ベンチャー企業が数多く誕生していますが、治療の片輪をなす医療用機器についてはまだまだ十分とは言い難い状況です。医療機器開発を成功させるには医工連携だけでなく、産学連携も欠かせません。リサーチユニット「先端医療技術・生体材料・医療機器臨床応用」は、つくば地区に集結しているこれらの知恵・技術と協調しつつ、臨床ニーズに基づく新規生体材料・医療機器・先端医療技術の開発と、その臨床応用に挑戦しています。
私たちの病気は、大きく分けて、投薬による内科的治療と手術による外科的治療の2つで治療されます。多くの画期的な新薬や治療器具が米国を中心とした大学の自由な風土を土壌に誕生していることを追い風に、日本でも大学の研究成果に礎を置く創薬系ベンチャー企業が数多く誕生していますが、治療の片輪をなす医療用機器についてはまだまだ十分とは言い難い状況です。医療機器開発を成功させるには医工連携だけでなく、産学連携も欠かせません。リサーチユニット「先端医療技術・生体材料・医療機器臨床応用」は、つくば地区に集結しているこれらの知恵・技術と協調しつつ、臨床ニーズに基づく新規生体材料・医療機器・先端医療技術の開発と、その臨床応用に挑戦しています。
よりよい外科技術を早期に臨床応用したい
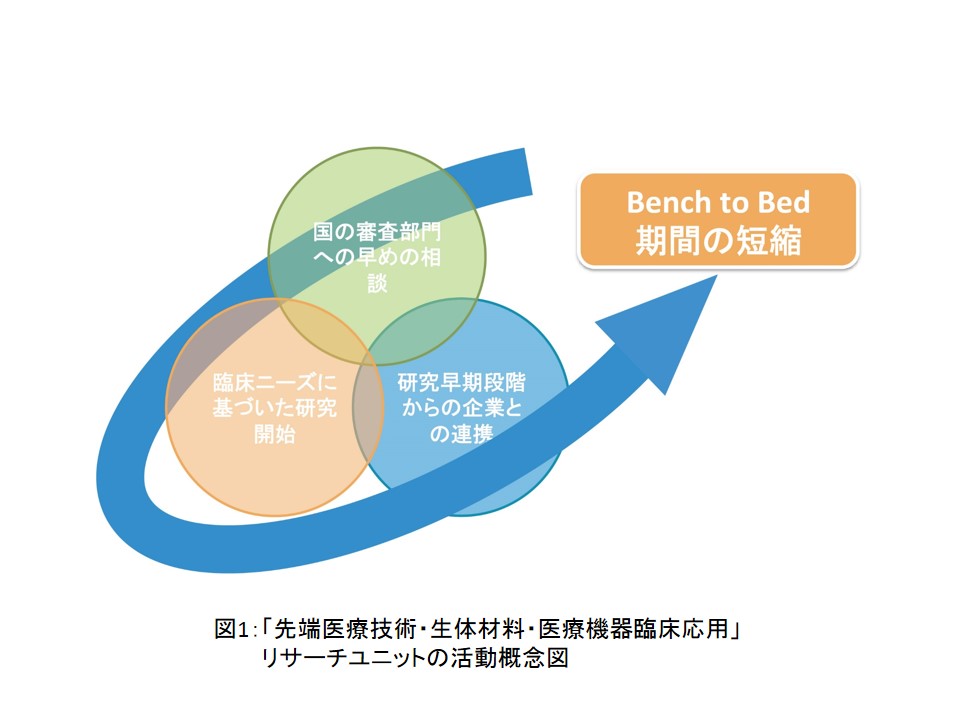
国内でも診断用医療機器の開発・製造は行われているのですが、治療用の医療機器に関しては90%以上が海外製品です。私は臨床医で外科医ですから、よりよい技術で患者を治したいという希望があるのですが、新しい医療機器の開発には長い時間と多額のお金がかかります。また、臨床医の協力、審査機関との連携も欠かせません。そこで、本リサーチユニットでは、Bench to Bed、つまり、研究の成果が臨床の現場で活用されるまでのタイムラグをなるべく狭めるべく、「①臨床ニーズに基づいた研究開始」「②研究早期段階からの企業との連携」「③国の審査部門への早めの相談」の3つを軸に、新規生体材料・医療機器・先端医療技術の開発と、その臨床応用の促進に取り組んでいます (図1)。
機器と手術法を組み合わせ、日本の新しい治療技術を世界へ
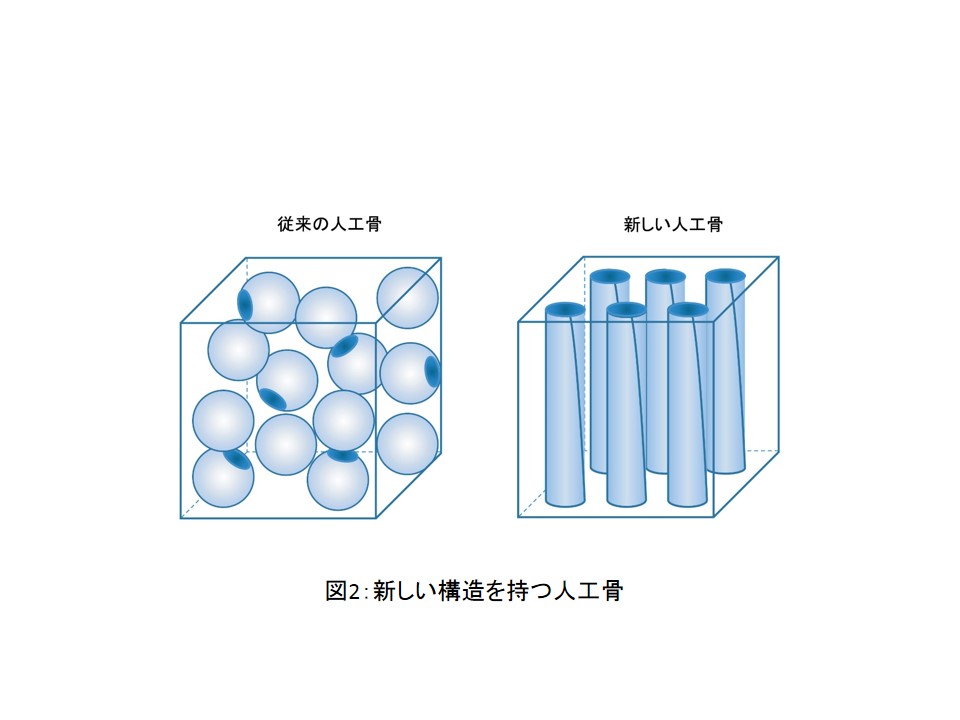
最終ゴール=自分たちで開発した新しい技術での治療、にはまだ時間がかかりそうですが、徐々に成果もでています。たとえば、クラレ、筑波大学、物質・材料研究機構の3者で2009年に開発した新しい人工骨は新規医療素材として販売中です。この人工骨の新しいところは材質ではなく一軸連通孔構造なのですが (図2)、最近、この人工骨について新知見が得られたので、臨床応用範囲が広がっています。
日本では治療法・手術法に対する特許が認められていないので、特許申請は機器と治療法のセットになりますが、海外には治療法・手術法で申請しています。特許と産学連携の両輪で日本発の新しい材料や医療技術、手術道具などを世界に発信していくとともに、このような活動に興味を持つ若い人たちが増えるといいな、と思っています。
社会への貢献・実績
● 充填物保持部材、充填物保持部材の製造方法および骨修復キットに関する特許の出願
● 医工連携フォーラム2014の主催(図3)
● 第5回CREILセンター公開シンポジウムの開催(2013年2月14日)
(取材:平成25年9月19日)
Development of Original Japanese Treatment Techniques using Patent and Academic-Industrial Collaboration
Unit members : Kanamori Akihiro Nozawa Daisuke Yanagi Kenichi
Key words: medicine-engineering collaboration, biomaterial, regulatory science
Patients’ diseases are usually treated with drugs and/or surgery. With the background of the fact that a number of revolutionary drugs and medical devices have been developed in universities overseas, mainly in the US, partially owing to their unique nature, many venture companies targeting drug discovery have been established based on the results of studies performed by universities in Japan. However, the field of medical devices, which play important roles in disease treatment, is still underdeveloped. To invent  medical devices, it is essential to achieve not only medicine-engineering collaboration, but also academic-industrial collaboration. Our research unit, named the “Development and Clinical Application of Emerging Medical Technology, Biomaterial, and Medical Devices”, aims to develop and clinically apply advanced biomaterials, medical devices, and medical technologies based on clinical needs, using the wisdom and techniques of researchers of Tsukuba City.
medical devices, it is essential to achieve not only medicine-engineering collaboration, but also academic-industrial collaboration. Our research unit, named the “Development and Clinical Application of Emerging Medical Technology, Biomaterial, and Medical Devices”, aims to develop and clinically apply advanced biomaterials, medical devices, and medical technologies based on clinical needs, using the wisdom and techniques of researchers of Tsukuba City.
Desire for early clinical application of better surgical techniques
In Japan, medical devices for diagnostic purposes are being developed and manufactured, but more than 90% of those designed for disease treatment are from overseas. As a clinician and surgeon, I have a strong desire to treat patients with better techniques, but it takes a long time and large amount of money to develop novel medical devices. Cooperation with clinicians and review boards is also essential. Given this situation, to minimize the time from the achievement of study results to their application to clinical settings, so-called “bench-to-bed”, our research unit is working to develop advanced biomaterials, medical devices, and medical technologies, and promote their clinical application with the ideas of: 1) initiation of research based on clinical needs; 2) cooperation with companies from the early stages of the research; and 3) early consultation with an investigative department of the government (Figure 1).
Worldwide release of novel Japanese treatment techniques consisting of medical devices and surgical methods
Our goal is to treat diseases using our new techniques. It will take a certain period of time to achieve this goal, but we are increasingly developing useful products. For example, in 2009, Kuraray Co., Ltd, the University of Tsukuba, and the National Institute for Materials Science developed artificial bone as a novel medical product that is currently on sale. Its main characteristic is not a material, but a structure with unidirectionally interconnected pores (Figure 2), and the clinical application of such bones is becoming increasingly prevalent as new findings regarding them have recently been reported.

Figure 2: Artificial bone with a new structure
In Japan, because it is not possible to obtain a patent for treatment or surgical techniques, patents for treatment techniques must be applied for in combination with medical devices; however, we can apply for patents singly for treatment/surgical techniques in other countries. We hope that we will be able to release original Japanese medical techniques and materials (e.g., surgical instruments) worldwide using patent and academic-industrial collaboration, and that young researchers with an interest in such activities will increase.
Social contributions and achievements
● Applications for patents for components that support filling materials, the method for manufacturing such components, and bone-repairing kit
●Holding of the Medicine-Engineering Collaboration Forum 2014 (Figure 3)
●Holding of the 5th Symposium by the CREIL Center (February 14, 2013)



